Fistreem Knowledge
Water purification removes contaminants from water, including biological contaminants, suspended solids and gases.
Tap water contains numerous contaminants and even trace impurity levels, which, while fine to drink, can affect various scientific experiments. To get good, reliable, accurate scientific data from experiments, it is vital that you use purified water for making up buffers or rinsing glassware.
We at Fistreem International have designed economic water purification systems that guarantee pure water for all your laboratory needs.
Dissolved Gases
What are they?
The leading gases dissolved in purified water are oxygen, nitrogen, carbon dioxide, and traces of inert gases, all in equilibrium with ambient air.
Where do they come from?
Dissolved oxygen, nitrogen, and inert gases will pass through reverse osmosis membranes.
Which Applications do they affect?
Dissolved gases can alter the pH of laboratory water and upset ionic balance. High concentrations of dissolved gases can cause bubble formation hampering flow through columns, e.g. HPLC.
Inorganic Salts
The most common inorganic impurities are sodium, calcium, iron, magnesium, chloride, sulphate and nitrate ions residuals. Bicarbonate ions are produced by dissolved atmospheric CO2 in the product water due to environmental exposure.
Where do they come from?
They are also released from ion exchange media within the purification system as the media capacities are used.
Which Applications do they affect?
Higher concentrations of less soluble species can lead to the degradation in spray characteristics for ICP-MS and AAS and can degrade column and detector performance in HPLC.
Micro-organisms and Biomolecules
What are they?
They are commonly released by biofilms within the water purification system. Pseudomonas, Flavobacterium and Acinetobacter are typically detected in waters.
Where do they come from?
Bacteria, viruses, fungi and algae are found in most surface waters and can also multiply in the pipes delivering water to its point of use.
Which Applications do they affect?
Bacteria and their degradation, such as endotoxins, can interfere with many bioprocesses, including PCR, clinical analysis and testing.
Organic Compounds
What are they?
The most common organic impurities are residuals of low molecular weight organics in feed water. Organic impurities can be produced by bacteria within the system.
Where do they come from?
Organics can be released from ion exchange and other media within the purification system.
Which Applications do they affect?
Higher concentrations of less soluble species can lead to the degradation in spray characteristics for ICP-MS and AAS and can degrade column and detector performance in HPLC.
Particulates
What are they?
Water will contain suspended matter, including hard particles (e.g. sand, rock, slit and pipework debris), soft particles (vegetal debris) and colloidal particles (organic and non-organic) between 1-10µM.
Where do they come from?
Most particles within the water system will come from biofilms and those shed by ion exchange resins, other media, tubing and fittings.
Which Applications do they affect?
Particulates can build up and cause blockages in any system with fine tubing, filters, atomisers, pumps, LC columns and detectors, causing issues with HPLC, ICP-MS etc.
Pyrogens
What are they?
Pyrogens are predominately endotoxins. The significant component of endotoxins is lipopolysaccharides derived from Gram-damaging bacteria walls.
Where do they come from?
They are commonly released by biofilms within the water purification system.
Which Applications do they affect?
Failure of techniques requiring sterile conditions and poor results from molecular biology techniques can cause filters and tubing blocking.
Technologies
There are several different methods of water purification that you can use to create pure water. The technology best suited for your needs depends on the application you require, the grade of water needed and the level of impurities in your water.
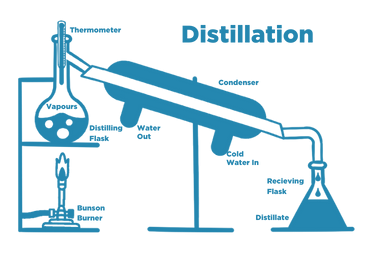
Distillation
Distillation is a long-established method that separates water from contaminants by changing the state of water from a liquid phase to a gas phase and then back to a liquid phase.
Water is first heated to boiling, and water vapour rises to a condenser. Cooling water lowers the temperature, so the vapour is condensed, collected, and stored.
Distillation removes the following water impurities
- Organic compounds
- Inorganic salts
- Microrganisms and biomolecules
- Pyrogens
Reverse Osmosis (RO)
In the osmosis process, a solvent naturally moves from a low solute concentration (high water potential) through a membrane to a place of high solute concentration (low water potential).
Reverse osmosis, an applied pressure, is used to overcome osmotic pressure. The solute is retained on the membrane’s pressurised side, and the pure solvent can pass to the other side.
Reverse Osmosis removes the following water impurities
- Organic compounds
- Inorganic salts
- Microorganisms and Biomolecules
- Pyrogens
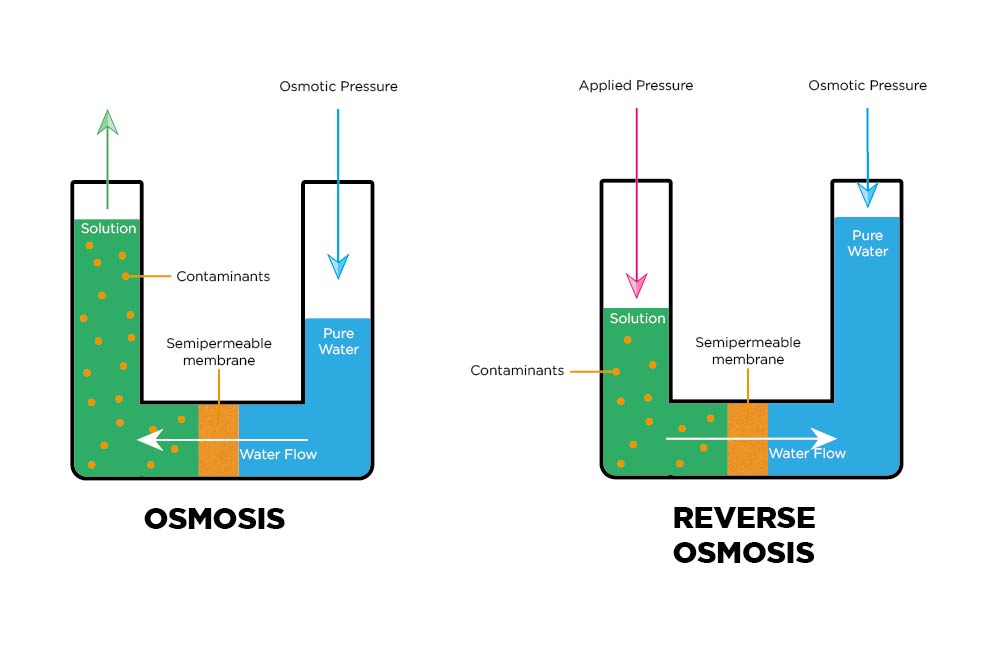
Reverse Osmosis (RO)
In the osmosis process, a solvent naturally moves from a low solute concentration (high water potential) through a membrane to a place of high solute concentration (low water potential).
Reverse osmosis, an applied pressure, is used to overcome osmotic pressure. The solute is retained on the membrane’s pressurised side, and the pure solvent can pass to the other side.
Reverse Osmosis removes the following water impurities
- Organic compounds
- Inorganic salts
- Microorganisms and Biomolecules
- Pyrogens
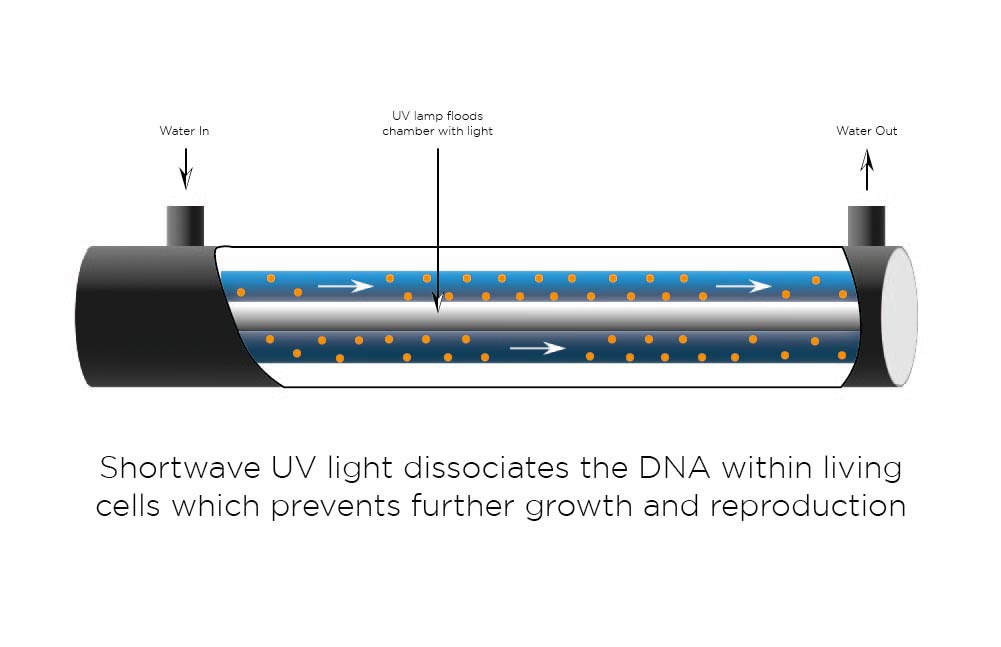
Ultra-Violet (UV)
UV is used to disinfect water. Two wavelengths are commonly used, 185nm and 254nm.
254nm UV has a robust bacterial action as it damages DNA and RNA polymerase at low doses preventing replication. UV light at 185nm has a strong oxidising action which breaks down large organic molecules into smaller ionised components, ultimately CO2.
A UV chamber typically consists of a UV lamp mounted in a quartz tube in the centre of a stainless-steel tube. Water then flows through the zone between the quartz and the steel tubes.
Ultra-Violet removes the following water impurities:
- Trace organic compounds
- Inactivates micro-organisms and biomolecules