Antibiotics are vital medications that are used for killing bacteria. They manage this with specific targeting techniques that result in the death of bacterial cells while not affecting human cells. Antibiotics can work in a whole host of different methods by targeting bacteria-specific structures, including the cell wall ribosomes, and interfering with bacterial DNA. A typical example is penicillin, which interferes with the bacterial cell wall, causing it to function incorrectly, resulting in the death of the bacterial cell.
Antibiotic testing can be helpful in both clinical and research settings. Clinicians will use this type of testing to help them decide which antibiotics would be most suitable for treating a patient and the dosage level. In research, this test is practical when developing new antibiotics and analysing how effective the candidates are against certain target strains of bacteria.
The disc diffusion method of analysis is the most common method used and is a quick and inexpensive way to analyse antimicrobial activity with respect to a target organism. This method was gradually developed throughout the 20th century, but in the 1950s, it became apparent that it needed to be standardised. This standardisation can be accredited to W. Kirby and A. Bauer, whose development was approved by the World Health Organisation in 1961 and was appropriately named the Kirby-Bauer method.
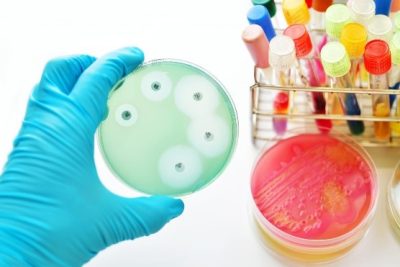
The method involves infusing small paper discs with a specific antibiotic and then placing them onto an agar plate that has been thoroughly spread with a bacterium of interest using a sterile swab. Common ways of putting the discs are:
-
- Six arranged in a ring formation on a 90mm petri dish
- In a grid formation on square dishes
Various antibiotic discs can be used on one single plate to save time, expenses, and resources. Once the discs are placed on the plate, the antibiotic begins to diffuse across the agar. As the antibiotic diffuses out further over the agar, its concentration is reduced. The plates are then incubated for around 18 to 24 hours, but this all depends on the bacteria on the plate, the antibiotic used, and the laboratory protocols.
Once the dishes have finished incubating, the plates can be analysed. The area around each of the antibiotic discs in which the antibiotic is inhibiting the growth of the bacteria is called the “zone of inhibition”. The concentration becomes weaker as the antibiotic diffuses further from the disc. At a certain point, the antibiotic will become so weak that it can no longer inhibit the bacterium’s growth, giving a visible boundary at the edge of the zone. The diameter of the zone is indicative of how great the sensitivity of the bacterium is against the antibiotic. The larger the zone size, the more effective the antibiotic is against that specific bacterium. If there is no zone surrounding the antibiotic, this means that this bacterium is resistant to the antibiotic. It is then possible to compare the zone sizes against a standardised chart, which can determine whether the bacteria are sensitive, resistant, or have intermediate sensitivity to the bacterium.
Currently, most laboratories use the method of measuring inhibition zones by manually measuring the zones using callipers or rulers. This method can be time-consuming, inaccurate and imprecise, particularly when the standardised charts for comparing zone sizes are highly specific, with small margins between bacteria being sensitive or not sensitive to antibiotics.
Synbiosis can offer customers products that make inhibition zone measuring quick, easy, and accurate, with results that are repeatable time and time again. The ProcScan is the optimum choice for inhibition zone measurement, with its scanning technology at 600dpi providing clear and high-quality images. Coupled with the ProtoCOL software, it can count multiple zones at once while maintaining high accuracy, measuring each zone to within 50µm within seconds. The ProtoCOL 3 and the ChromaZona both have the capability to measure inhibition zones, giving the user a selection of devices to cater for their exact needs and price range. Zones can also be manually edited by a user after the initial measurement with x500 zoom on the image, allowing a user complete control over where the inhibition zone ends.
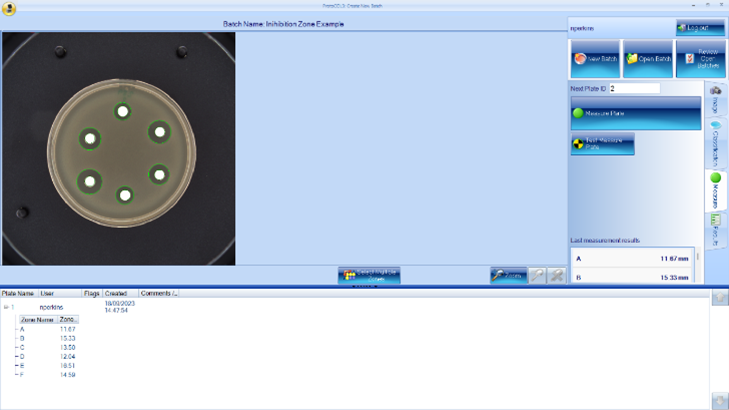
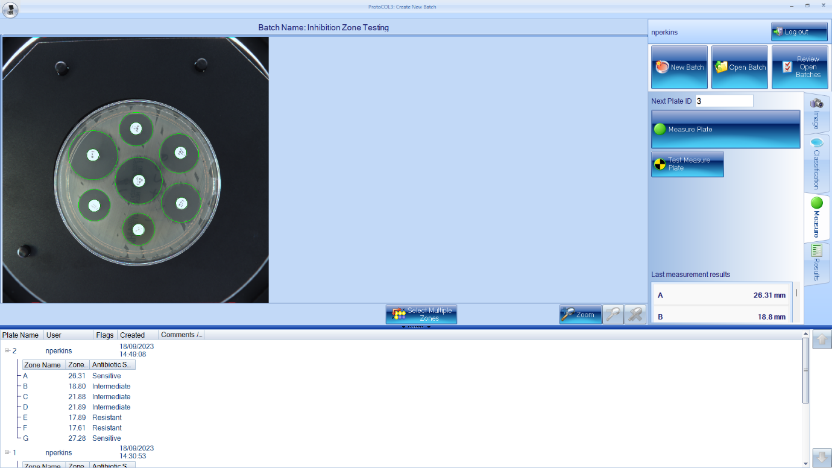
We also offer extra software modules, including “Antibiotic Susceptibility Testing”, which has EUCAST and CLSI guidelines incorporated, allowing a user to select the antibiotic and bacterium of choice during setup so the results can be compared against these breakpoints and give a “Sensitive”, “Intermediate”, or “Resistant” result, making the process much more streamlined and efficient. These devices are 21 CFR part 11 compliant and contain audit trails for various fields, from user log-ins to any manual edits made to a plate. All the data of the inhibition zones can be made into customisable reports through the ProtoCOL software in PDF, Excel or CSV format to suit your personal needs. They can even be linked up to a LIMS for easily displayed results.
If you have any questions or want more information regarding our range of products, please contact me at nick.perkins@synoptics.co.uk or any of my colleagues at sales@synbiosis.com. Our product brochures are also available on our website, www.synbiosis.com.
